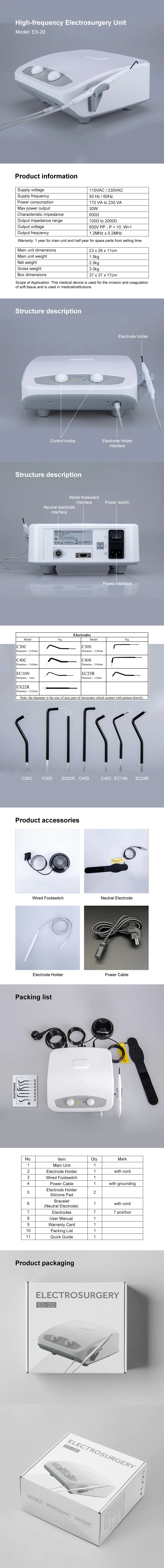
⭐This device is used for the fast and precise incision and coagulation of soft tissue and is used in medical institutions.
⭐Safe/Professional/Effective/Convenient.
⭐Incision and coagulation can be adjusted freely.
⭐Seven pieces high performance and hyperfine electrodes.
⭐Reliable and compact unit.
⭐Excellent therapeutic control.
⭐The whole set of electrode holder is light and autoclavable.
⭐Innovative conductive bracelet (Neutral Electrode).
⭐30 watts is enough for greater efficiency.
Model: ES-20
Supply voltage: 115VAC / 230VAC
Supply frequency: 50 Hz / 60Hz
Power consumption: 170 VA ~ 230 VA
Max power output: 30W
Characteristic impedance: 600Ω
Output impedance range: 100Ω ~ 2000Ω
Output voltage: 650V PP - P = 10, W=1
Output frequency: 1.2MHz ± 0.2MHz
Main unit dimensions: 23 x 26 x 11cm
Main unit weight: 1.5kg
Net weight: 2.0kg
Gross weight: 3.0kg
Box dimensions: 37 x 37 x 17cm
Operating temperature: +10℃ to +30℃
Storage temperature: -20℃ to +70℃
Operating relative humidity: 30% to 75%
Storage relative humidity: 10% to 100% including condensation
Atmospheric pressure: 80KPa to 106KPa
Altitude: ≤2000 meters
1 x Main Unit
1 x Electrode Holder(with cord)
1 x Wired Footswitch
1 x Power Cable(with grounding)
2 x Electrode Holder Silicone Pad
1 x Bracelet (Neutral Electrode)
7 x Electrodes
1 x User Manual
1 x Warranty Card
1 x Packing List
1 x Quick Guide
Seller name: Dreamland Company
City, State: Beijing, China
Regulation Number: 872.4200
Classification Product Code: EFB
Subsequent Product Code: EGS























